Why Supercritical CO2?
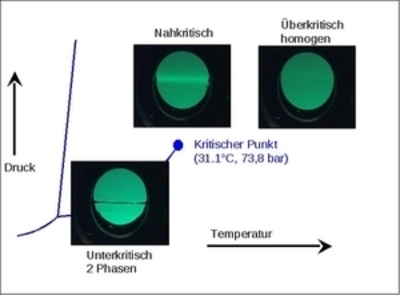
Supercritical CO2 is used in natural products extraction on an industrial scale since it replaces conventional solvents such as halogenated hydrocarbons and offers a series of additional advantages. Along the vapour pressure curve of a pure substance, the liquid phase and vapour are in equilibrium. At the critical point, the vapour phase ends. The supercritical range lies above this point.
In the supercritical range, the thermophysical properties change substantially. In a homogeneous phase, i.e., without phase boundary as transport resistance, the density of the supercritical fluid can be varied continuously via changes in temperature and pressure in such a way that when the density is similar to that of a liquid the transport properties and thus the mass and heat transfer are the same as in the gas phase. This is why extractions or reactions are carried out in supercritical CO2. The separation of the product is then carried in the 2-phase range. The extract is free of solvent residues and has a constant product quality.
